One Minute Read: Comprehensive Labelling and Annotation
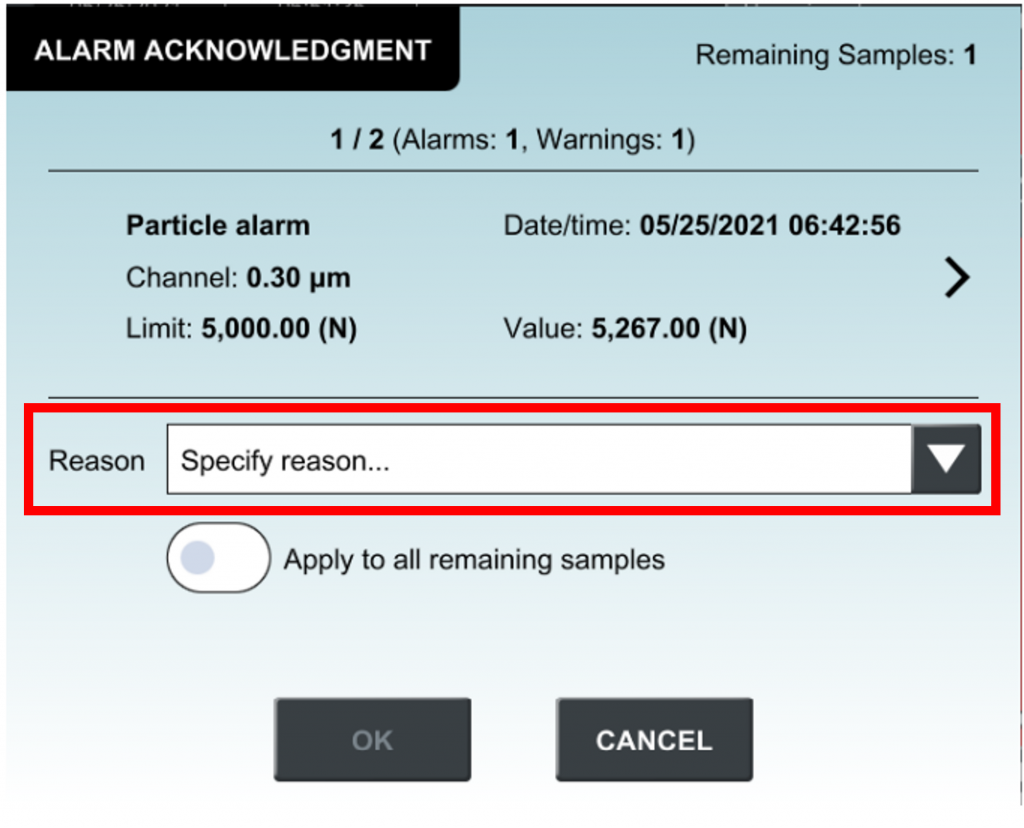
Too often, we add handwritten notes onto the particle count report generated from our particle counter because the default information presented on the report is simply not comprehensive enough. However, as we add useful information onto the report, we also invite questions and comments from cleanroom auditors because this practice is regarded as a threat to data integrity according to FDA’s 21 CFR Part 11.
How can we avoid these problems once and for all, then?
Simply by using the Lasair® Pro aerosol particle counter series! – With the new feature that allows users to annotate additional information such as cleanroom type and location – all on the particle counter, and ahead of sampling or even post sampling, Lasair® Pro ensures data integrity as all information entered and stored on the counter is not alterable, hence complying with the 21 CFR Part 11.
But does this mean extra workload as there will be more information to key into the counter?
Not at all thanks to the NFC (Near Field Communication) technology! By pre-setting and uploading specific recipes onto different NFC cards, users can now ensure data integrity and save time from configuring their particle counter when sampling using the Lasair® Pro aerosol particle counter!
